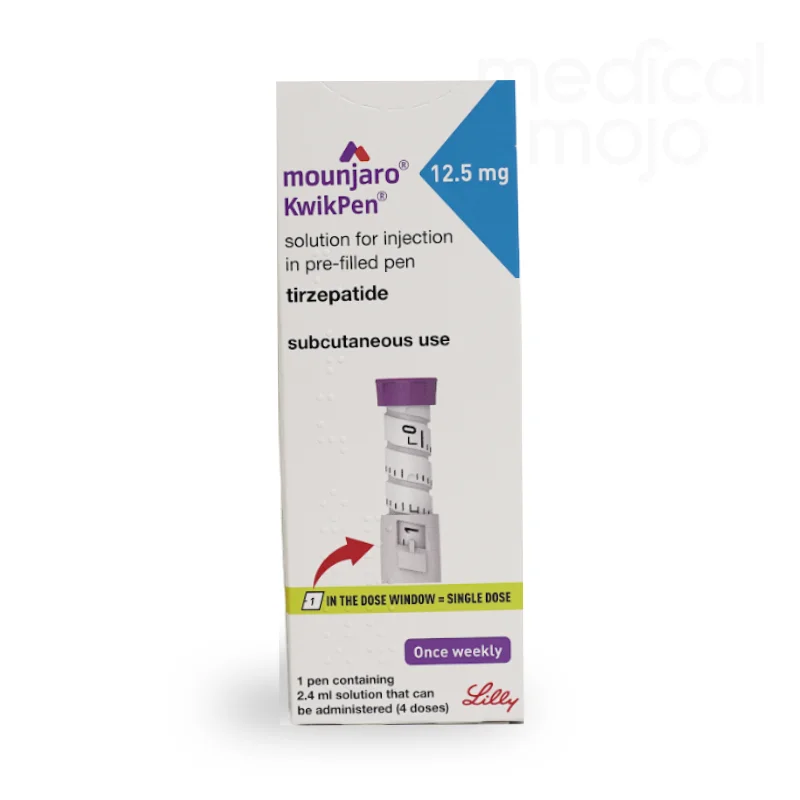
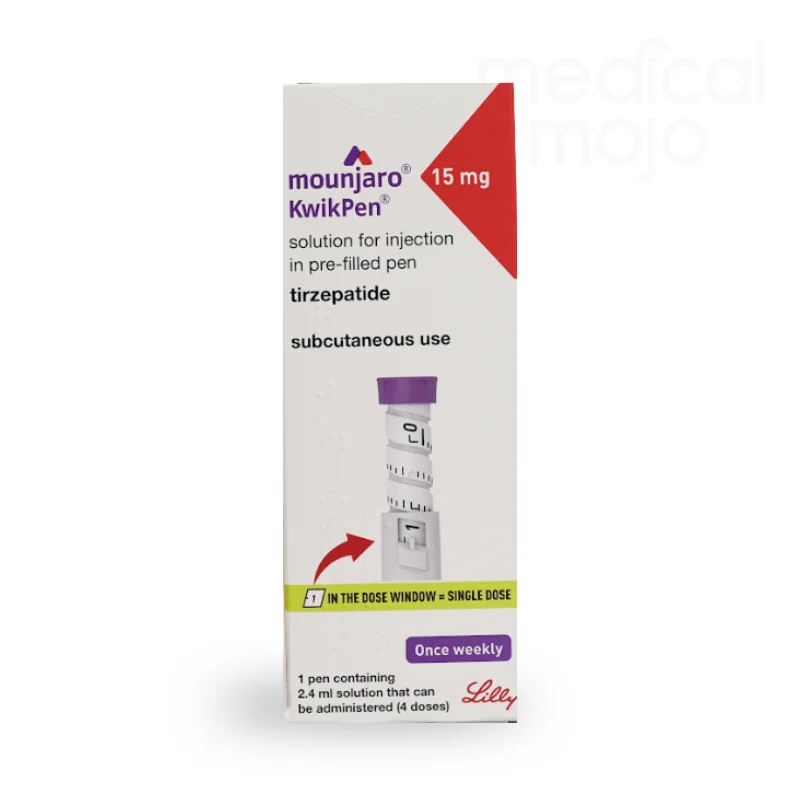
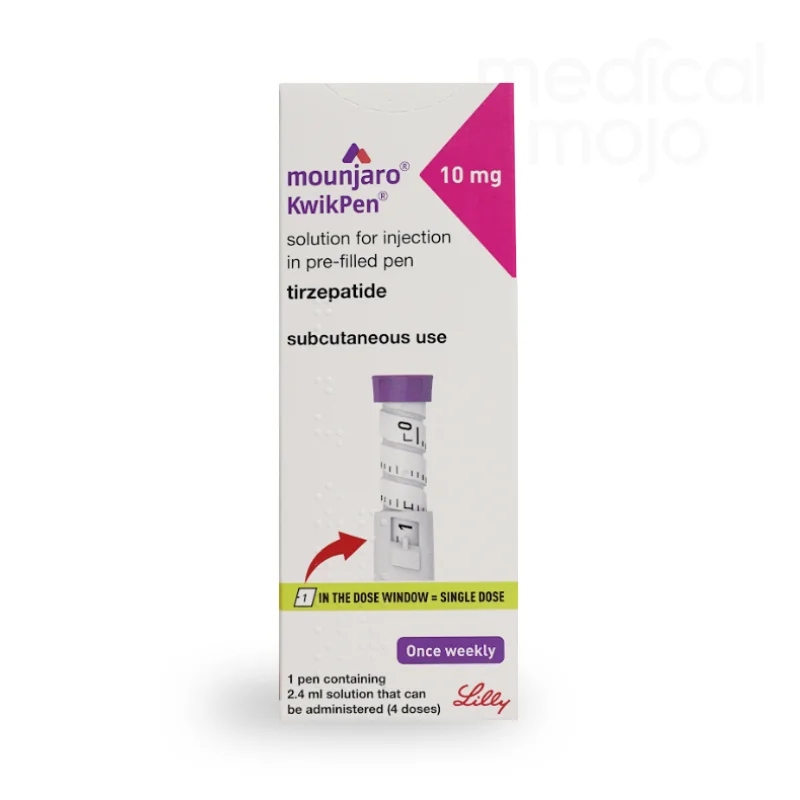
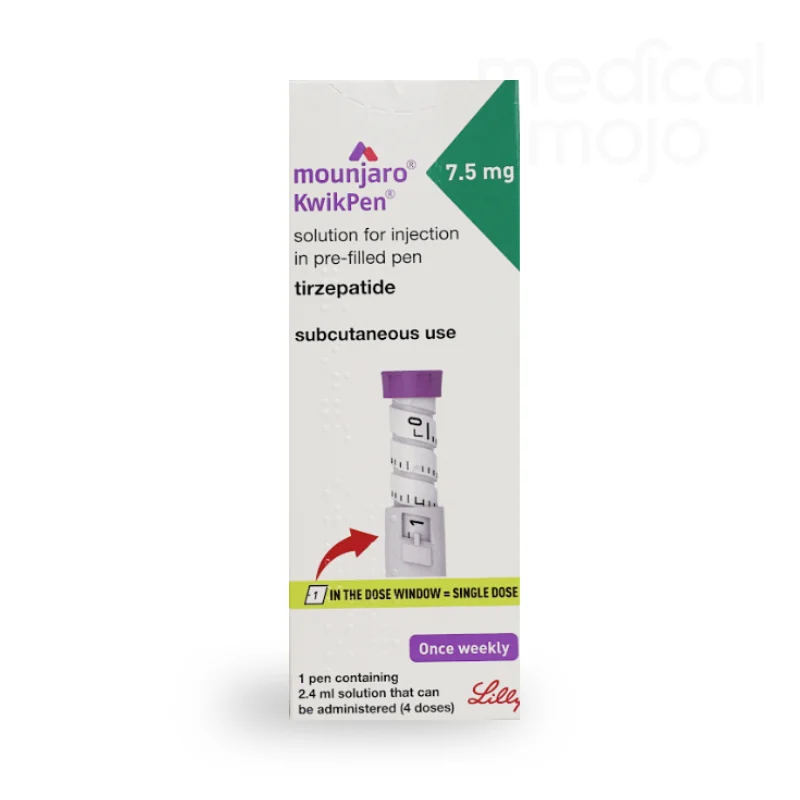
What is Mounjaro 12.5mg injection?
Mounjaro 12.5mg is a weight loss treatment available as a pre-filled pen for once-weekly injections. It is the UK-licensed version of the US-approved weight loss injection, Zepbound.
The active ingredient in Mounjaro is Tirzepatide, which binds to the receptors of two hormones, GIP and GLP-1. These hormones are naturally produced by your body in response to eating. By attaching to these receptors, Tirzepatide mimics the actions of GIP and GLP-1, signaling your body to increase insulin release from the pancreas. This helps control blood sugar levels and sends signals of fullness to the brain, reducing appetite and preventing cravings, ultimately aiding in weight loss.
What is Mounajro 12.5mg injection used for?
For weight loss, Mounjaro 12.5mg is also used along with a reduced-calorie diet and increased physical activity in adults who:
- Have a BMI of 30 kg/m² or greater (obesity), or
- Have a BMI between 27 kg/m² and 30 kg/m² (overweight) and have weight-related health problems (such as prediabetes, type 2 diabetes, high blood pressure, abnormal blood fat levels, obstructive sleep apnea, or a history of heart attack, stroke, or blood vessel problems).
BMI (Body Mass Index) measures your weight in relation to your height.
How do you use Mounjaro 12.5mg injection?
Always use this medicine exactly as your doctor or pharmacist has instructed. If you are unsure, check with your doctor or pharmacist.
Each KwikPen contains 4 doses of Mounjaro, available in 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg.
How Much to Use:
- The starting dose is 2.5 mg once a week for four weeks. After four weeks, your doctor will increase your dose to 5 mg once a week.
- Your doctor may increase your dose by 2.5 mg increments to 7.5 mg, 10 mg, 12.5 mg, or 15 mg once a week if needed. Your doctor will instruct you to stay on each dose for at least 4 weeks before increasing to a higher dose.
It's important to note that everyone is different, and there is no rush to increase your dose. You can stay at a dose for an extra month or two, or even permanently, if you prefer. If you need to decrease the dose due to side effects or if you're not tolerating a higher dose well, that's perfectly fine.
Only increase your dose if you are tolerating your current dose well (i.e., no side effects) and wish to enhance your weight loss.
If you're satisfied with your weight loss and not experiencing side effects, you can maintain your current dose for as long as necessary. Typically, maintenance doses are 5mg, 10mg, or 15mg.
If you experience side effects, consider staying at your current dose or reducing it. Speak to our clinical team for further advice.
Choosing When to Administer Mounjaro:
- You can use your pen at any time of the day, with or without meals. Try to use it on the same day each week. To help you remember, you may want to mark the day on a calendar or use the “Instructions for Use” to tick off the day of the week when you inject your first dose.
- If necessary, you can change the day of your weekly Mounjaro injection, as long as it has been at least 3 days since your last injection. After selecting a new dosing day, continue with once-a-week dosing on that new day.
How to Inject Mounjaro KwikPen:
- Mounjaro is injected under the skin (subcutaneous injection) of your stomach area (abdomen), upper leg (thigh), or upper arm. You may need help from someone else if you choose to inject in your upper arm.
- You can use the same area of your body each week, but be sure to choose a different injection site within that area each time. If you also inject insulin, choose a different injection site for the insulin.
- To inject Mounjaro, follow these steps to take your dose:
- Familiarize yourself with the complete guide that comes with the pen.
- Remove the pen from the packaging and check the expiration date.
- Ensure you have a clean, dry area of skin on your stomach, thigh, or upper arm.
- Remove the cap from the pen and clean the injection site with an alcohol swab.
- Select a new pen needle. Always use a new needle for each injection to prevent infections and blockages.
- Attach the needle to the pen by pushing it straight on and twisting until tight.
- Remove the outer needle shield and keep it for later use.
- Hold the pen at a 90-degree angle against your skin and press the button on top to release the needle.
- Hold the pen in place for at least 5 seconds, then release the button to retract the needle.
- Dispose of the used pen and needle in a sharps container or follow your healthcare professional's disposal instructions.
Mounjaro is taken weekly. Ensure you take it at the same time each week and rotate injection sites to prevent skin irritation.
Who should not take Mounjaro 12.5mg injection?
Do not use Mounjaro KwikPen:
- If you are allergic to tirzepatide or any other ingredients in this medicine, check the product information leaflet below.
Warnings and Precautions:
Talk to your doctor, pharmacist, or nurse before using Mounjaro if:
- You have severe digestion problems or delayed stomach emptying (including severe gastroparesis).
- You have ever had pancreatitis (inflammation of the pancreas causing severe, persistent stomach and back pain).
- You have eye problems (such as diabetic retinopathy or macular edema).
- You are using a sulfonylurea (another diabetes medicine) or insulin, as Mounjaro can cause low blood sugar (hypoglycemia). Your doctor may need to adjust your doses of these medications to reduce this risk.
When starting treatment with Mounjaro, you might experience dehydration due to vomiting, nausea, and/or diarrhea, which can affect kidney function. It is important to drink plenty of fluids to avoid dehydration. Contact your doctor if you have any concerns.
Children and Adolescents: This medicine should not be given to children and adolescents under 18 years of age as it has not been studied in this age group.
Pregnancy: This medicine should not be used during pregnancy as its effects on an unborn child are unknown. If you are pregnant, think you may be pregnant, or are planning to have a baby, consult your doctor before using this medicine. It is recommended to use contraception while using Mounjaro.
If you are a woman with obesity or overweight and using oral contraceptives, consider also using a barrier method (e.g., a condom) or switching to a non-oral contraceptive method for 4 weeks after starting Mounjaro and for 4 weeks after each dose increase.
Breast-feeding: It is unknown whether tirzepatide passes into breast milk. A risk to newborns/infants cannot be ruled out. If you are breast-feeding or planning to breast-feed, consult your doctor before using this medicine. You and your doctor should decide whether to stop breast-feeding or delay using Mounjaro.
Driving and Using Machines: It is unlikely that this medicine will affect your ability to drive and use machines. However, if you use Mounjaro in combination with a sulfonylurea or insulin, low blood sugar (hypoglycemia) may occur, which can reduce your ability to concentrate. Avoid driving or using machines if you experience any signs of low blood sugar, such as headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, fast heartbeat, and sweating. Refer to section 4 for more details. For information on the increased risk of low blood sugar, see section 2, ‘Warnings and Precautions.’ Consult your doctor for further information.
Mounjaro KwikPen Contains Sodium: This medicine contains less than 1 mmol sodium (23 mg) per dose, essentially making it ‘sodium-free.’
Mounjaro KwikPen Contains Benzyl Alcohol: Each multiple-dose pre-filled pen contains 5.4 mg benzyl alcohol [E1519] in each 0.6 ml dose. Benzyl alcohol may cause allergic reactions. Consult your doctor or pharmacist for advice if you have liver or kidney disease, as large amounts of benzyl alcohol can build up in your body and may cause side effects (called “metabolic acidosis”).
What are the side effects with Mounjaro 12.5mg injection?
Like all medicines, this medication can cause side effects, although not everyone will experience them.
Serious Side Effects
Uncommon (may affect up to 1 in 100 people):
- Inflamed pancreas (acute pancreatitis): This can cause severe pain in the stomach and back that does not go away. Seek immediate medical attention if you experience these symptoms.
Rare (may affect up to 1 in 1,000 people):
- Severe allergic reactions (e.g., anaphylactic reaction, angioedema): Seek immediate medical help and inform your doctor if you experience symptoms such as breathing problems, rapid swelling of the lips, tongue, and/or throat with difficulty swallowing, and a fast heartbeat.
Other Side Effects
Very Common (may affect more than 1 in 10 people):
- Low blood sugar (hypoglycemia) when tirzepatide is used for the treatment of type 2 diabetes with medicines that contain a sulfonylurea and/or insulin: If you are using a sulfonylurea or insulin, the dose may need to be lowered while you use tirzepatide. Symptoms of low blood sugar may include headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, fast heartbeat, and sweating. Your doctor should tell you how to treat low blood sugar.
- Feeling sick (nausea)
Other Side Effects
Very Common (may affect more than 1 in 10 people):
- Diarrhea
- Vomiting (usually goes away over time)
- Constipation
These side effects are usually not severe and are most common when first starting tirzepatide but tend to decrease over time in most patients. Constipation and vomiting are very common when used for weight management, but common when used for type 2 diabetes.
Common (may affect up to 1 in 10 people):
- Low blood sugar (hypoglycemia) when tirzepatide is used for type 2 diabetes with both metformin and a sodium-glucose co-transporter 2 inhibitor (another diabetes medicine): Symptoms may include headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, fast heartbeat, and sweating. Your doctor should tell you how to treat low blood sugar.
- Allergic reaction (hypersensitivity): Symptoms may include rash, itching, and eczema.
- Dizziness observed in patients treated for weight management.
- Low blood pressure observed in patients treated for weight management.
- Decreased appetite observed in patients treated for type 2 diabetes.
- Stomach (abdominal) pain
- Indigestion (dyspepsia)
- Stomach bloating
- Burping (eructation)
- Gas (flatulence)
- Reflux or heartburn (gastroesophageal reflux disease – GORD): A condition caused by stomach acid coming up into the tube from your stomach to your mouth.
- Hair loss observed in patients treated for weight management.
- Feeling tired (fatigue)
- Injection site reactions: Such as itching or redness.
- Fast pulse: Common when used for type 2 diabetes and uncommon when used for weight management.
- Increased levels of pancreatic enzymes (such as lipase and amylase) in blood: Increased levels of amylase is uncommon in weight management.
Uncommon (may affect up to 1 in 100 people):
- Low blood sugar (hypoglycemia) when tirzepatide is used with metformin for type 2 diabetes: Symptoms may include headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, fast heartbeat, and sweating. Your doctor should tell you how to treat low blood sugar.
- Gallstones
- Weight loss observed in patients treated for type 2 diabetes
- Injection site pain
- Increased calcitonin levels in blood
- Cholecystitis (infection of the gallbladder) observed in patients treated for weight management.
Reporting of Side Effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at
www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. By reporting side effects, you can help provide more information on the safety of this medicine.
Does Mounjaro 12.5mg interact with any other medicines?
Tirzepatide can slow down stomach emptying, which may impact how other oral medications are absorbed. This is most noticeable when you first start taking tirzepatide.
Paracetamol:
- When taken with tirzepatide, the peak concentration (Cmax) of paracetamol is reduced by 50%, and it takes an additional hour to reach this peak (tmax).
- No dose adjustment for paracetamol is needed.
- The effect on paracetamol absorption is dose-dependent. Lower doses of tirzepatide (0.5 and 1.5 mg) only slightly change paracetamol levels. After four weekly doses of tirzepatide (5/5/8/10 mg), there is no effect on paracetamol Cmax and tmax, and overall exposure (AUC) remains unchanged.
Oral Contraceptives:
- A single 5 mg dose of tirzepatide can reduce the Cmax and AUC of oral contraceptives, delaying their peak levels.
- Ethinyl estradiol: Cmax reduced by 59%, AUC by 20%, with a delay in tmax of 4 hours.
- Norelgestromin: Cmax reduced by 55%, AUC by 23%, with a delay in tmax of 4.5 hours.
- Norgestimate: Cmax reduced by 66%, AUC by 20%, with a delay in tmax of 2.5 hours.
- These changes are not considered clinically significant, so no dose adjustment is needed for women with a normal BMI.
Special Considerations for Oral Contraceptives in Overweight or Obese Women:
- There is limited data on how tirzepatide affects oral contraceptives in overweight or obese women. Reduced effectiveness cannot be ruled out.
- It is recommended to switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks when starting tirzepatide and after each dose increase.