Mounjaro and other GLP-1 (glucagon-like peptide-1) receptor agonists, such as Wegovy and Ozempic, are widely used for managing type 2 diabetes and promoting weight loss. These medications work by mimicking gut hormones to stimulate insulin release, lower blood sugar levels, and reduce appetite. But does Mounjaro cause thyroid cancer? This was an initial concern due to early to early rodent studies with GLP-1 agonists. However, new research suggests that the actual risk in humans may be minimal. This blog will attempt to answer the question that everyone has when starting Mounjaro, does Mounjaro cause cancer?
Before we discuss the evidence, we will first go over some of the basics about Mounjaro.
Use the navigational table below to skip to topics of interest.
Table of contents
- What is Mounjaro (Tirzepatide)?
- How effective is Mounjaro?
- Mounjaro weight loss table
- The six strengths of Mounjaro
- What Is thyroid medullary cancer?
- Warnings about GLP-1 medications and thyroid cancer
- Why the risk differs between rodents and humans
- New study finds low risk of thyroid cancer with GLP-1
- What does the evidence say about the link between Mounjaro and thyroid cancer?
- What should patients do?
- Lose weight with Mounjaro and personalised support
-
 Mounjaro 2.5mg Injection£145.99 – £565.99
Mounjaro 2.5mg Injection£145.99 – £565.99 -
 Mounjaro 10mg Injection£185.99 – £735.99
Mounjaro 10mg Injection£185.99 – £735.99 -
 Mounjaro 15mg Injection£229.99 – £899.99
Mounjaro 15mg Injection£229.99 – £899.99
What is Mounjaro (Tirzepatide)?
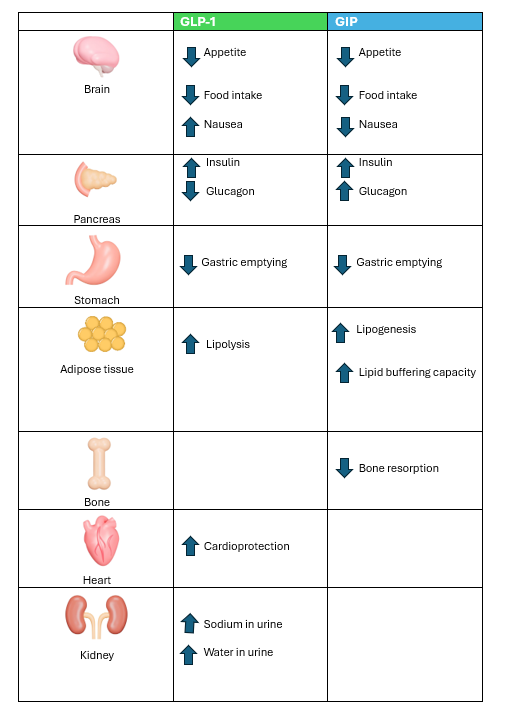
Mounjaro is a once-weekly injectable medication developed by Eli Lilly. The active ingredient, tirzepatide, is a groundbreaking treatment combining two gut hormones—glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).
These hormones, known as incretins, stimulate insulin release in response to food intake. Because Mounjaro has both GLP-1 and GIP properties, it is known as a “twincretin”.
Incretins like GLP-1 and GIP contribute to weight loss by:
Early research on diet-induced obese (DIO) mice showed that combining GLP-1 and GIP resulted in significantly greater weight loss . Recognising its potential, Eli Lilly patented tirzepatide in 2016 and later validated its effectiveness in humans through the SURMOUNT trials.
The table below highlights the effects of GLP-1 and GIP.
How effective is Mounjaro?
The SURMOUNT 1 trial assessed the weight-loss potential of tirzepatide in non-diabetic individuals with obesity or those who were overweight. Participants included those with:
- A body mass index (BMI) of 30 kg/m² or higher, or
- A BMI of 27 kg/m² or higher with at least one weight-related condition, such as high blood pressure.
Over 72 weeks, participants taking the highest dose of tirzepatide (15 mg) lost an average of 22.5% of their body weight, with nearly 90% achieving at least 5% weight loss. In comparison, the GLP-1-based medication semaglutide delivers an average weight loss of 12.4%. These findings highlight Mounjaro’s superior efficacy.
Mounjaro weight loss table
| Week | Tirzepatide (5mg) | Tirzepatide(10mg) | Tirzepatide (15mg) | Placebo |
| 0 | 105 kg | 105 kg | 105 kg | 105 kg |
| 4 | 101 kg | 100.5 kg | 100 kg | 104.5 kg |
| 8 | 96.5 kg | 95.5 kg | 95 kg | 104 kg |
| 12 | 93.5 kg | 91.5 kg | 90 kg | 103.5 kg |
| 16 | 91.5 kg | 89 kg | 87 kg | 103 kg |
| 24 | 90 kg | 86 kg | 84.5 kg | 102.5 kg |
| 36 | 89.3 kg | 84.5 kg | 83 kg | 102.4 kg |
| 48 | 89 kg | 83.5 kg | 82 kg | 102.4 kg |
| 72 | 88.6 kg | 82.6 kg | 81.2 kg | 102.4 kg |
Summary of Weight Loss Results from SURMOUNT 1
5 mg Tirzepatide: 16.4 kg (36.1 lbs)
10 mg Tirzepatide: 22.4 kg (49.4 lbs)
15 mg Tirzepatide: 23.8 kg (52.5 lbs)
Placebo: 2.6 kg (5.7 lbs)
Have questions about Mounjaro?
Get a FREE telephone consultation with one of our pharmacists, who will call you and help you understand what the right options are for you.
Call me about Mounjaro
The six strengths of Mounjaro
Mounjaro is available in pre-filled injection pens in the following strengths:
Mounjaro is administered weekly as a subcutaneous injection, typically in the abdomen, thigh, or upper arm. To learn how to safely adminsiter Mounjaro, read our blog, “How to inject Mounjaro”.
What Is thyroid medullary cancer?
Medullary thyroid cancer (MTC) is a rare and more aggressive form of thyroid cancer that develops in specialised cells of the thyroid gland called parafollicular C cells. These cells produce calcitonin, a hormone that regulates calcium levels in the body. Unlike more common thyroid cancers, MTC is often associated with genetic mutations, particularly in the RET proto-oncogene, and it can occur in two ways:
- Sporadically (randomly, without a family history).
- Inherited, as part of genetic conditions like Multiple Endocrine Neoplasia Type 2 (MEN-2).
Symptoms of MTC may include:
- A noticeable lump in the neck.
- Trouble swallowing or speaking.
- Elevated levels of calcitonin detected through blood tests.
Although MTC is less common, early detection and appropriate management are crucial due to its potential to spread more aggressively than other thyroid cancers.
Warnings about GLP-1 medications and thyroid cancer
Warnings about the potential risk of medullary thyroid cancer developing with GLP-1 use came in pre-clinical studies in rodents. It was found that high doses of GLP-1 medications led to thyroid tumors in rodents. These tumors are typically preceded by increased calcitonin levels in the blood and abnormal growth of C cells in the thyroid (C-cell hyperplasia).
In humans, medullary thyroid cancer is often associated with mutations in the RET proto-oncogene. When researchers tested liraglutide, a GLP-1 agonist, in mice, it did not activate RET, a key driver of human C-cell cancers. Instead, liraglutide’s effects on rodent C cells were linked to a different signalling pathway involving the activation of a protein called mammalian target of rapamycin (mTOR), independent of RET or other common cancer pathways like MAPK.
These findings highlight that the risks observed in rodents may not directly apply to humans due to differences in biology, such as lower GLP-1 receptor activity in human thyroid cells.
Unlike rodents, humans and primates have very few or no GLP-1 receptors on their thyroid C cells, suggesting that these findings may not apply to people. This species-specific response in rodents highlights that the thyroid effects seen in these animals are unlikely to have the same relevance to humans.
Why the risk differs between rodents and humans
In rodents, thyroid tumors were linked to GLP-1 drugs because these animals have a higher density of GLP-1 receptors on their thyroid cells. Overactivation of these receptors led to tumour formation in the studies. Unlike rodents, humans and primates have very few or no GLP-1 receptors on their thyroid C cells, suggesting that these findings may not apply to people.
New study finds low risk of thyroid cancer with GLP-1
A study published in BMJ on April 10, 2024, provides reassurance. A large Scandinavian study examined whether GLP-1 receptor agonists, used for diabetes and weight loss, increase thyroid cancer risk. Comparing over 145,000 GLP-1 users with patients on other diabetes medications (DPP4 and SGLT2 inhibitors), the study found no significant increase in thyroid cancer risk over an average follow-up of 3.9 years. In the GLP-1 group (145,410 participants), thyroid cancer occurred at a rate of 1.33 events per 10,000 person-years.
What does the evidence say about the link between Mounjaro and thyroid cancer?
While studies raise concerns about a potential association between GLP-1 medications, like Mounjaro, and thyroid cancer, no conclusive evidence has confirmed this link in humans. Animal studies showed an increased risk of MTC due to GLP-1 receptor activity in rodents, but this risk is unlikely to apply to humans because of biological differences.
What should patients do?
If you are taking Mounjaro or similar medications, here are some key steps:
- Continue treatment as prescribed: The benefits of GLP-1 medications in managing diabetes and promoting weight loss outweigh the theoretical risks for most patients.
- Discuss concerns with your Doctor: If you have a personal or family history of MTC or MEN-2, inform your healthcare provider before starting or continuing treatment.
- Monitor for symptoms: Be vigilant for signs like lumps in the neck or difficulty swallowing, and report them promptly to your doctor.
Are weight loss treatments making you tired, or have they led to a sudden increase in hair loss? Do you struggle with sleep?
Get a free month’s supply of one of our compounded treatments for energy, hair loss or sleep, with your first purchase of Mounjaro or Wegovy from Medical Mojo.
Claim your FREE offer
Lose weight with Mounjaro and personalised support
Mounjaro is not just about medication; pairing it with expert coaching can significantly enhance weight loss outcomes. A recent study found that participants receiving personalised coaching achieved greater weight loss compared to those without coaching, with the most noticeable results seen over 12 months.
At Medical Mojo, we combine Mounjaro with tailored weight loss support to help you succeed. Our program includes:
- Weekly progress reviews and motivational support.
- Expert advice on setting realistic and achievable goals.
- Personalised coaching tailored to your weight loss goals.
- FREE needles and sharps bins, ensuring a seamless start to your treatment.
| Week | Tirzepatide (5mg) | Tirzepatide(10mg) | Tirzepatide (15mg) | Placebo |
| 0 | 231 lbs | 231 lbs | 231 lbs | 231 lbs |
| 4 | 222.2 lbs | 221.3 lbs | 220.5 lbs | 230 lbs |
| 8 | 212.7 lbs | 210.5 lbs | 209.4 lbs | 229 lbs |
| 12 | 206 lbs | 201.6 lbs | 198.4 lbs | 228 lbs |
| 16 | 201.6 lbs | 196.2 lbs | 191.8 lbs | 227 lbs |
| 24 | 198.4 lbs | 189.6 lbs | 186.2 lbs | 226 lbs |
| 36 | 196.8 lbs | 186.3 lbs | 183 lbs | 225.8 lbs |
| 48 | 196.2 lbs | 184 lbs | 180.7 lbs | 225.8 lbs |
| 72 | 195.3 lbs | 182.1 lbs | 179 lbs | 225.8 lbs |
-
 Mounjaro 12.5mg Injection£229.99 – £899.99
Mounjaro 12.5mg Injection£229.99 – £899.99 -
 Mounjaro 7.5mg Injection£185.99 – £735.99
Mounjaro 7.5mg Injection£185.99 – £735.99 -
 Mounjaro 5mg Injection£145.99 – £565.99
Mounjaro 5mg Injection£145.99 – £565.99
Start your weight loss journey today with Medical Mojo.
Let Medical Mojo help you achieve the weight-loss results you’ve been striving for. With expert guidance, individualised plans, and the right resources, successful weight loss has never been more attainable. Take the first step today and see how we can support your journey to a healthier, happier you.
Disclaimer: This article is for informational purposes only and is not a substitute for professional medical advice.
References
- Boucai, L., Zafereo, M. and Cabanillas, M.E., 2024. Thyroid cancer: a review. Jama, 331(5), pp.425-435.
- Bokvist, B.K.; Coskun, T.; Cummins, R.C.; Alsina-Fernandez, J. GIP and GLP-1 Co-Agonist Compounds. U.S. Patent 947478, 25 October 2016
- Coskun, T., Sloop, K.W., Loghin, C., Alsina-Fernandez, J., Urva, S., Bokvist, K.B., Cui, X., Briere, D.A., Cabrera, O., Roell, W.C. and Kuchibhotla, U., 2018. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Molecular metabolism, 18, pp.3-14.
- Madsen, L.W., Knauf, J.A., Gotfredsen, C., Pilling, A., Sjögren, I., Andersen, S., Andersen, L., Sietske de Boer, A., Manova, K., Barlas, A. and Vundavalli, S., 2012. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology, 153(3), pp.1538-1547.
- Pasternak, B., Wintzell, V., Hviid, A., Eliasson, B., Gudbjörnsdottir, S., Jonasson, C., Hveem, K., Svanström, H., Melbye, M. and Ueda, P., 2024. Glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer: Scandinavian cohort study. bmj, 385.
- Rosol, T.J., 2013. On-target effects of GLP-1 receptor agonists on thyroid C-cells in rats and mice. Toxicologic pathology, 41(2), pp.303-309.
- Roy, M., Chen, H. and Sippel, R.S., 2013. Current understanding and management of medullary thyroid cancer. The Oncologist, 18(10), pp.1093-1100.
- Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. J Obes Metab Syndr. 2023 Mar 30;32(1) –
- Unick, J.L., Pellegrini, C.A., Dunsiger, S.I., Demos, K.E., Thomas, J.G., Bond, D.S., Lee, R.H., Webster, J. and Wing, R.R., 2024. An Adaptive Telephone Coaching Intervention for Patients in an Online Weight Loss Program: A Randomized Clinical Trial. JAMA Network Open, 7(6), pp.e2414587-e2414587.


