The battle of the weight-loss medicines. The first blows in the most anticipated heavyweight battle have already been thrown, and no we are not talking about boxing and a certain fight in December. It’s the battle of the weight-loss giants Mounjaro versus Wegovy that has taken the spotlight in the pharmaceutical industry.
Initial results from the latest SURMOUNT-5 trial show that Mounjaro’s US equivalent weight-loss version, Zepbound, outperformed Wegovy in head-to-head studies, delivering 47% greater weight loss. Additionally, results from the SURPASS-2 trial demonstrate Mounjaro’s superior efficacy in managing weight and blood sugar levels in patients with type 2 diabetes, adding to its reputation as a versatile treatment for obesity and related conditions. So, in the final analysis of Mounjaro versus Wegovy although Mounjaro has superior weight loss benefits Wegovy remains a strong contender with its proven cardiovascular benefits.
Before we dive into the study’s details we will just go over some of the basics about Mounjaro and Wegovy.
Use the navigational table below to skip to topics of interest.
Table of contents
- What is an incretin?
- What is Mounjaro (Tirzepatide)?
- How effective is Mounjaro?
- Mounjaro weight loss table
- What strengths of Mounjaro are available?
- What is Wegovy?
- How effective is Wegovy for weight loss?
- Comparison of GLP-1 and GIP effects
- Mounjaro versus Wegovy the results
- Additional evidence for better weight-loss results with Mounjaro
- The consequence of the SURPASS-5 trial
- Novo Nordisk’s response to Mounajro: CagriSema
- Key advantages of Mounjaro and Wegovy
- Where to buy Mounjaro and Wegovy?
- Loosing weight with Mounjaro and personalised support
-
 Mounjaro 2.5mg Injection£145.99 – £565.99
Mounjaro 2.5mg Injection£145.99 – £565.99 -
 Mounjaro 10mg Injection£185.99 – £735.99
Mounjaro 10mg Injection£185.99 – £735.99 -
 Mounjaro 15mg Injection£229.99 – £899.99
Mounjaro 15mg Injection£229.99 – £899.99
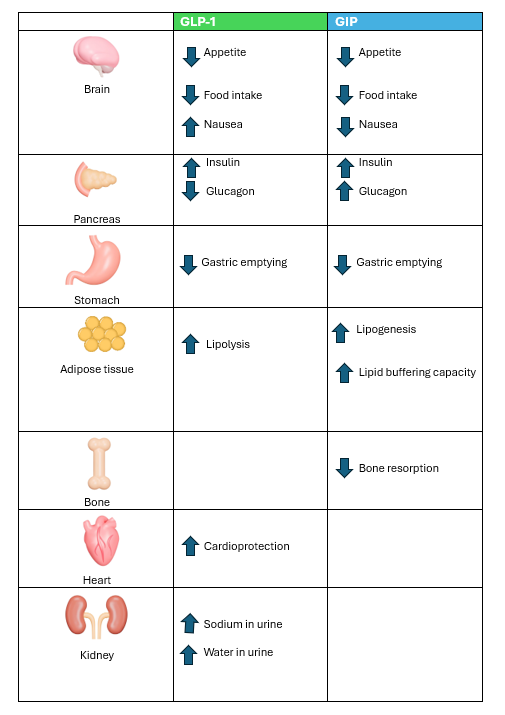
What is an incretin?
Both Mounjaro and Wegovy work by mimicking the actions of chemical substances in the body called incretins. Researchers in the early 1970s first discovered incretins—hormones in the human body that stimulate insulin release from beta cells.
These hormones, produced in the intestines, play a vital role in stimulating insulin released from the pancreas, that is they “increase” the levels of insulin in response to food in the gut. This effect occurs because nutrients from food trigger the release of incretin hormones, which stimulate the pancreas to produce insulin (Nauck, M.A et al., 2016).
The two most well-known incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (Modi P 2007) (Tupas, G.D., et al 2020).
GLP-1 and GIP are released by specialised cells in the intestines, called enteroendocrine cells, in response to food intake. These hormones play a crucial role in regulating metabolism after meals.
What is Mounjaro (Tirzepatide)?
Mounjaro, contains the active ingredient tirzepatide and is marketed as Zepbound in the US for weight loss, is a once-weekly injectable medication developed by Eli Lilly. This groundbreaking medication is the first to combine two gut hormones—glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)—both of which stimulate insulin release in response to food intake. Because it stimulates the release of two incretins it has been called a “twincretin.”
Incretins like GLP-1 and GIP work to reduce weight by
- appetite suppression,
- increasing satiety, and
- slowing gastric emptying (Sinha R et al., 2023).
However, when researchers discovered that the combination of GLP-1 and GIP caused more weight-loss in diet induced obese (DIO) mice (Coskun T et al., 2018), they knew they were onto a winner. Eli Lily had already applied for the patent in 2016 (Bokvist, B.K et al., 2016), now all they had to do was prove it worked to cause the same weight loss in humans. This is what the SURMOUNT trials set out to achieve.
How effective is Mounjaro?
The SURMOUNT 1 trial evaluated the weight loss potential of Mounjaro (tirzepatide) in non-diabetic individuals. Participants included those with a body mass index (BMI) of 30 kg/m² or higher, or 27 kg/m² or higher with at least one weight-related condition, such as high blood pressure.
Over the course of 72 weeks, participants taking the highest dose of Mounjaro (15 mg) experienced an average weight loss of 22.5% of their body weight, with nearly 90% of participants achieving at least a 5% weight loss.
In comparison the GLP-1-based medication, semaglutide, which achieves an average weight loss of 12.4%, falls short of the outcomes seen with Mounjaro (Wilding JPH et al., 2021).
The results from the SURMOUNT 1 trial are summarised in the table below.
Mounjaro weight loss table
| Week | Tirzepatide (5mg) | Tirzepatide(10mg) | Tirzepatide (15mg) | Placebo |
| 0 | 105 kg | 105 kg | 105 kg | 105 kg |
| 4 | 101 kg | 100.5 kg | 100 kg | 104.5 kg |
| 8 | 96.5 kg | 95.5 kg | 95 kg | 104 kg |
| 12 | 93.5 kg | 91.5 kg | 90 kg | 103.5 kg |
| 16 | 91.5 kg | 89 kg | 87 kg | 103 kg |
| 24 | 90 kg | 86 kg | 84.5 kg | 102.5 kg |
| 36 | 89.3 kg | 84.5 kg | 83 kg | 102.4 kg |
| 48 | 89 kg | 83.5 kg | 82 kg | 102.4 kg |
| 72 | 88.6 kg | 82.6 kg | 81.2 kg | 102.4 kg |
Studies reveal that the highest maintenance dose of Mounjaro, 15mg per week, can support weight loss of up to 22.5% of a person’s starting body weight.
Summary of total weight loss after 72 weeks with Mounjaro
5 mg Tirzepatide: 16.4 kg (36.1 lbs)
10 mg Tirzepatide: 22.4 kg (49.4 lbs)
15 mg Tirzepatide: 23.8 kg (52.5 lbs)
Placebo: 2.6 kg (5.7 lbs)
Have questions about Mounjaro?
Get a FREE telephone consultation with one of our pharmacists, who will call you and help you understand what the right options are for you.
Call me about Mounjaro
What strengths of Mounjaro are available?
Mounjaro is available in pre-filled injection pens with the following strengths:
Mounjaro is administered weekly as a subcutaneous injection, typically in the abdomen, thigh, or upper arm. To learn how to safely adminsiter Mounjaro, read our blog, “How to inject Mounjaro”.
Mounjaro timeline
United States
May 2022: Approved by the FDA for T2DM treatment under the brand name Mounjaro. (Drugs.com 2022)
November 2023: Approved by the FDA for chronic weight management under the brand name Zepbound. (Drugs.com)
United Kingdom
October 2022: Granted UK marketing authorization for T2DM treatment under the name Mounjaro. (Pulse Today 2022)
November 2023: Approved by the MHRA for weight loss and weight management in adults with obesity (BMI ?30 kg/m²) or overweight (BMI 27–30 kg/m²) with weight-related conditions. (MHRA 2023)
What is Wegovy?
Wegovy, developed by Novo Nordisk, is another once-weekly injectable medication. Unlike Mounjaro, Wegovy focuses solely onGLP-1 receptor agonism, reducing appetite and slowing digestion to promote weight loss (Naslund E et al., 1999), ( Flint A et al., 1998).
How effective is Wegovy for weight loss?
The STEP (Semaglutide Treatment Effect in People with obesity) 1 trial which investigated weight loss with the GLP-1 Wegovy 2.4mg or semaglutide in adults with obesity or who were overweight without diabetes. The STEP-1 trial found that participants lost an average of 14.9% of body weight with Wegovy compared to 2.4% with placebo (Wilding, J.P et al.,2021).
Comparison of GLP-1 and GIP effects
| Week | Tirzepatide (5mg) | Tirzepatide(10mg) | Tirzepatide (15mg) | Placebo |
| 0 | 231 lbs | 231 lbs | 231 lbs | 231 lbs |
| 4 | 222.2 lbs | 221.3 lbs | 220.5 lbs | 230 lbs |
| 8 | 212.7 lbs | 210.5 lbs | 209.4 lbs | 229 lbs |
| 12 | 206 lbs | 201.6 lbs | 198.4 lbs | 228 lbs |
| 16 | 201.6 lbs | 196.2 lbs | 191.8 lbs | 227 lbs |
| 24 | 198.4 lbs | 189.6 lbs | 186.2 lbs | 226 lbs |
| 36 | 196.8 lbs | 186.3 lbs | 183 lbs | 225.8 lbs |
| 48 | 196.2 lbs | 184 lbs | 180.7 lbs | 225.8 lbs |
| 72 | 195.3 lbs | 182.1 lbs | 179 lbs | 225.8 lbs |
-
 Mounjaro 12.5mg Injection£229.99 – £899.99
Mounjaro 12.5mg Injection£229.99 – £899.99 -
 Mounjaro 7.5mg Injection£185.99 – £735.99
Mounjaro 7.5mg Injection£185.99 – £735.99 -
 Mounjaro 5mg Injection£145.99 – £565.99
Mounjaro 5mg Injection£145.99 – £565.99
Mounjaro versus Wegovy the results
The SURMOUNT-5 trial was first to directly compared Mounjaro (tirzepatide) and Wegovy (semaglutide) in 751 participants who were obese or overweight with weight-related health conditions, such as hypertension, sleep apnoea, or heart disease. Importantly, none of the participants had diabetes.
Key Results of the SURMOUNT 5 trial
- Mounjaro (Zepbound): Achieved an average weight loss of 20.2% of body weight over 72 weeks.
- Wegovy: Achieved an average weight loss of 13.7% of body weight over the same period.
These results highlight Mounjaro’s dual-hormone mechanism as a more effective approach to weight management compared to Wegovy’s single-pathway strategy.
Additional evidence for better weight-loss results with Mounjaro
An older study called the SURPASS-2 trial which compared Mounjaro (tirzepatide) against Wegovy (semaglutide) in patients with type 2 diabetes. The aim of this study was to compare the effect of weekly tirzepatide with weekly semaglutide 1mg on blood sugar levels in type 2 diabetics. However This trial provided further insights into Mounjaro’s benefits, particularly for diabetic populations struggling with obesity.
Key Weight Loss Results (SURPASS-2 Trial):
- Tirzepatide 5 mg: Average weight reduction of 7.6 kg (16.7 lbs).
- Tirzepatide 10 mg: Average weight reduction of 9.3 kg (20.5 lbs).
- Tirzepatide 15 mg: Average weight reduction of 11.2 kg (24.7 lbs).
- Semaglutide 1 mg: Average weight reduction of 5.7 kg (12.6 lbs).
Tirzepatide demonstrated significantly greater weight loss at all dose levels compared to semaglutide, with the Mounjaro 15 mg dose achieving almost double the weight reduction seen with semaglutide (Frías, J.P et al., 2021). However, this was not a like for like study, since the maintenance weight loss dose for Wegovy or semaglutide is 2.4mg weekly. However, despite that, even the lower doses of Mounjaro gave better weight loss results than the semaglutide 1mg.
These results demonstrate that Mounjaro is not only more effective for weight loss but also offers superior blood sugar control, making it a dual-purpose medication for patients with type 2 diabetes and obesity.
The consequence of the SURPASS-5 trial
The initial SURMOUNT-5 trial results have significantly boosted Eli Lilly’s market value, with the company adding $19 billion to its valuation following the recent findings. The U.S. drugmaker’s shares rose 2.5% to a three-week high, adding some $19 billion to Lilly’s market value and taking it over $790 billion, while Novo Nordisk’s Copenhagen-listed shares fell as much as 1.8% (Medscape. (2024).
Novo Nordisk’s response to Mounajro: CagriSema
Novo Nordisk is developing a new dual-action drug, CagriSema, which combines semaglutide with amylin, another gut hormone. Early trials suggest that CagriSema could achieve weight loss of up to 25%, potentially rivalling or surpassing Mounjaro. Late-stage trial results are expected soon, positioning Novo Nordisk to remain competitive (Reuters. 2024, November 6).
Key advantages of Mounjaro and Wegovy
Mounjaro (Tirzepatide/Zepbound) advantages
- Superior Weight Loss: Demonstrated 20.2% weight loss in head-to-head trials.
- Dual Mechanism: Combines GLP-1 and GIP action for enhanced results.
Wegovy (Semaglutide) advantages
- Cardiovascular Benefits: Proven to lower the risk of heart attack and stroke (Lincoff, A.M et al., 2023) and it was licensed in the UK to reduce reduce risk of serious heart problems in obese or overweight adults (MHRA 2024).
- Established Efficacy: Achieves an average weight loss of 15% in clinical trials.
Conclusion of Mounjaro versus Wegovy
Competition is healthy, especially in the pharmaceutical weight-loss industry, it has spurred innovation. We have witnessed groundbreaking reductions in weight, first with Wegovy producing around 15% weight reductions to 22.5% with Mounjaro. Now weight loss of up to 25% could be possible with the CagrisSema from NovoNordisk.
Losing weight has never been so easy, especially when you can order online after completing a health questionnaire.
Where to buy Mounjaro and Wegovy?
The most important thing to beat in mind when buying Mounjaro or Wegovy is that you buy from a registered pharmacy. When you buy your Mounjaro or Wegovy from Medical Mojo you have the reassurance that you are buying from a registered online pharmacy. What gives Medical Mojo an advantage is that their prescribers are experts in endocrinology and weight loss and are always available for a FREE weight loss consultation.
Are weight loss treatments making you tired, or have they led to a sudden increase in hair loss? Do you struggle with sleep?
Get a free month’s supply of one of our compounded treatments for energy, hair loss or sleep, with your first purchase of Mounjaro or Wegovy from Medical Mojo.
Claim your FREE offer
Loosing weight with Mounjaro and personalised support
A recent study highlighted the significant impact of personalised telephone coaching on weight loss. A recent study found that participants receiving personalised coaching achieved greater weight loss compared to those without coaching. Participants who received one-on-one coaching achieved substantially greater weight loss compared to those without coaching, with the most noticeable results occurring over a 12-month period.
Medical Mojo’s customised weight loss program
At Medical Mojo, we offer a dedicated weight loss coaching service designed to help you succeed. Our tailored program includes:
- Weekly check-ins to assess progress and maintain motivation.
- Expert advice to set realistic, achievable goals.
- Personalised coaching ensures you receive ongoing support throughout your journey.
- FREE needles and sharps bins, so you have everything you need to get started.
Start your weight-loss journey with Medical Mojo
Let Medical Mojo help you achieve the results you’ve been striving for. With expert guidance, individualised plans, and the right resources, successful weight loss has never been more attainable. Take the first step today and see how we can support your journey to a healthier, happier you.
Disclaimer: This article is for informational purposes only and is not a substitute for professional medical advice.
References
- Bokvist, B.K., Coskun, T., Cummins, R.C. and Alsina-Fernandez, J., Eli Lilly and Co, 2016. GIP and GLP-1 co-agonist compounds. U.S. Patent 9,474,780.
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009.
- Frías, J.P., Davies, M.J., Rosenstock, J., Pérez Manghi, F.C., Fernández Landó, L., Bergman, B.K., Liu, B., Cui, X. and Brown, K., 2021. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine, 385(6), pp.503-515.
- Lincoff, A.M., Brown-Frandsen, K., Colhoun, H.M., Deanfield, J., Emerson, S.S., Esbjerg, S., Hardt-Lindberg, S., Hovingh, G.K., Kahn, S.E., Kushner, R.F. and Lingvay, I., 2023. Semaglutide and cardiovascular outcomes in obesity without diabetes. New England Journal of Medicine, 389(24), pp.2221-2232.
- Medscape. (2024). Lilly’s Zepbound Tops Wegovy in Weight Loss Head-to-Head Trial. Retrieved from https://www.medscape.com/s/viewarticle/lillys-zepbound-tops-wegovy-weight-loss-head-head-trial-2024a1000m49?ecd=WNL_trdalrt_pos1_ous_241205_etid7056497&uac=453679SR&impID=7056497
- Modi, P. Diabetes beyond insulin: Review of new drugs for treatment of diabetes mellitus. Curr. Drug Discov. Technol. 2007, 4, 39–47.
- Reuters. (2024, November 6). Novo Nordisk says its experimental drug CagriSema has similar side effects. Retrieved from https://www.reuters.com/business/healthcare-pharmaceuticals/novo-nordisk-says-its-experimental-drug-cagrisema-has-similar-side-effects-2024-11-06/
- Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. J Obes Metab Syndr. 2023 Mar 30;32(1):25-45. doi: 10.7570/jomes22067. Epub 2023 Feb 8. PMID: 36750526; PMCID: PMC10088547.
- Tupas, G.D.; Otero, M.C.B.; Ebhohimen, I.E.; Egbuna, C.; Aslam, M. Chapter 8—Antidiabetic Lead Compounds and Targets for Drug Development. In Phytochemicals as Lead Compounds for New Drug Discovery; Egbuna, C., Kumar, S., Ifemeje, J.C., Ezzat, S.M., Kaliyaperumal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–141.
- Unick, J.L., Pellegrini, C.A., Dunsiger, S.I., Demos, K.E., Thomas, J.G., Bond, D.S., Lee, R.H., Webster, J. and Wing, R.R., 2024. An Adaptive Telephone Coaching Intervention for Patients in an Online Weight Loss Program: A Randomized Clinical Trial. JAMA Network Open, 7(6), pp.e2414587-e2414587.
- Wilding, J.P., Batterham, R.L., Calanna, S., Davies, M., Van Gaal, L.F., Lingvay, I., McGowan, B.M., Rosenstock, J., Tran, M.T., Wadden, T.A. and Wharton, S., 2021. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine, 384(11), pp.989-1002.


